We're intimately familiar with a vast array of pharmaceutical inspection processes.
We proudly serve leading pharmaceutical and biotech brands around the world.
We discover your unique needs and processes to align all deliverables with your compliance goals.
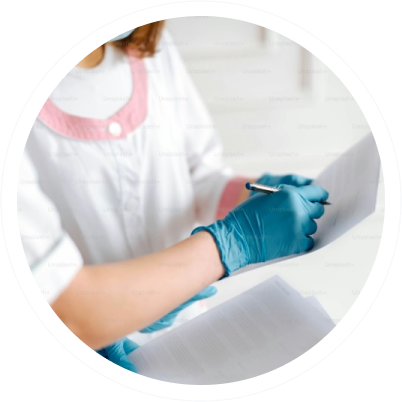
• Automatic Inspection Challenge Standards
• Parenteral Manual Inspection Defect Panels for Training (Defect Standards)
• Parenteral Fill Volume Standards
• Test Units for Protocol Execution

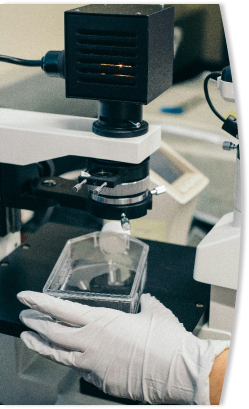
• Units Evaluation Using Magnification & Forensic Analysis
• Glass Breakage Pattern Recognition
• Particle Presence Confirmation & ID
• Inspector Training, Certification, Defect Familiarization & Recognition
• Knapp Studies
We execute the review and approval of equipment charts from CIP Skids, Ultrasonic, Parts Washers, and Autoclaves as evidence per status. Approval of corrective and preventative maintenance through the use of MAXIMO software is included.
We execute review and approval of test scripts (test runs and technical reports) intended to establish the equipment functionality and/or to define critical process parameters. This includes the evaluation of data and reports from studies and SOPs.
We develop and/or revise quality operation procedures for the oversight of the dispensing, buffer prep, formulation, and component prep areas.
We develop a training module for quality specialists supporting future commercial operations.



• Validation Project Management
• Construction/Qualification Project Management
• Product Transfer Project Management
• Vendor Qualification & Inspection of Suppliers Contract Manufacturing
• Validation Master Planning
• Facility & Utility Commissioning & Validation
• Validation of the Equipment Validation Process
• Computer Systems Validation
• Cleaning Validation
• Re-Qualification
• Standard Operating Procedures Preparation
• Laboratories Methods Validation
• Quality Assurance Systems Gap Analysis
• Quality Assurance Systems Investigations
• Quality Assurance For Corrective & Preventive Actions (CAPA)
• Quality Assurance Systems Technical Writing
• Development Training & Education In GMPs, GLPs & 21CFR Part 11

Let's talk to gain a clear understanding of your goals, processes, and parenteral visual inspection program needs.

Based on data and process configurations, we'll work with you to plan a visual inspection program that aligns with your goals.

We develop custom challenge sets and provide guidance and training to implement all relevant injectables visual inspections and studies.

Our visual inspection experts guide you through manual inspection, automatic inspection, inspection qualification, probability of detection, and beyond.


